Rubber Egg Science Experiment
Disclosure: This blog contains affiliate links which I may earn a small commission from if you purchase through them, at no extra cost to you.
The rubber egg experiment is a classic science experiment that can be used to demonstrate the chemical reaction of vinegar and calcium carbonate.
We love watching the magic, or science happen, over the course of the few days the reaction takes.
Every couple of hours, I’ll catch one of the kids peering over the benchtop on their tippy toes to see if there are any noticeable changes. It’s egg-specially exciting!
Just quietly, I have been known to have a sticky beak and give the experiment a little poke myself.
What can I say? Curiosity is a powerful desire to learn.

Rubber Egg Science Experiment
The rubber egg experiment is a fun and easy science activity to do with your kids.
It’s a great way for children to learn some of the basics of chemistry, including how acids react with calcium-based materials like eggs shells.
By dissolving the shell of the egg with vinegar, the inner contents of the egg are revealed – creating a naked egg!
Yup- an egg with no shell!
This simple experiment can be used to introduce students to basic scientific concepts such as diffusion and osmosis. That is, all while using just a couple of things found in the kitchen!
What you need to set up the rubber egg science experiment
- Container
- Egg
- Vinegar
The first time we did this science experiment presented lots of learning curves. The main one that stood out was the size of the container.
When selecting your container, consider the size of the egg and that the egg needs to be fully submerged with vinegar.
We used a glass jar. It was perfect as the glass was transparent.
We had a full view of the entire process. However, the jar turned out to be too small.
As the egg swelled and the gases amounted beneath the egg, something unexpected happened.
It was totally un-egg-spected!
The egg rose up out of the jar.
Yes, our experiment was making its way out of the jar.
With a gentle wiggle to allow for the gases underneath to escape, our experiment nestled back in quickly enough.
Interestingly enough the edges of the egg that made contact with the jar hadn’t disoved as much as those fully exposed to the vinegar.
That being said, we transferred it to a slightly wider jar to prevent the egg from making another unexpected move.

How to make a bouncy egg
How to set up your rubber egg science experiment
- Make some observations of the egg. Consider the size, hardness of the shell and the shape of the egg.
- Place egg gently into the container
- Pour the vinegar into the container to cover the egg
- Make observations. Are bubbles forming around the egg?
- Leave the egg for up to a week, changing the vinegar every few days, making observations each day
- Gently rinse the by product off the outside of the egg
- Make observations of the end result
- Explore the rubber egg!
What is the Rubber Egg Science Experiment
The rubber egg science experiment is a classic demonstration of how vinegar can dissolve the shell of an egg.
The process literally happens before your eyes. Well, that is over a few days.
There is no guessing whether the experiment is working or not. The reaction is clearly visible with a display of bubbles forming as a key indicator of the process in action.
This reaction occurs because the vinegar, containing acetic acid, reacts with calcium carbonate in the shell to produce carbon dioxide. The carbon dioxide is the bubbles that are seen forming around the egg!
When the vinegar and eggshell meet, carbon dioxide is released via a chemical reaction, causing the shell of the egg to dissolve.
Over a few days, the shell becomes no more.
Hence a naked egg!

What is the science behind the rubber egg experiment
The rubber egg experiment can be used to introduce students to basic scientific concepts such as diffusion and osmosis.
In addition, students can learn about the chemical reaction that takes place when vinegar is added to calcium carbonate.
The rubber egg experiment is a good demonstration of the effect osmosis has on the movement of water across a membrane.
The eggshell is porous.
This means the eggshell contains tiny holes. Through these tiny holes, water can move from inside to outside the egg and vice versa.
This happens via osmosis.
Osmosis occurs when two substances exist on either side of a semi-permeable membrane which are of different concentrations.
To make both sides the same, osmosis occurs.
That is, solvents or water pass through the semi-permeable membrane to reach equilibrium. That means they would be at the same concentration.
After soaking for a few days, the rubber egg is clearly plumper.
This is due to the extra water that has crossed over the membrane, to the inside of the egg.
How Does Osmosis Work?
Osmosis is the diffusion of water across a semi-permeable membrane.
Semi-permeable means that some small particles can diffuse through the membrane but others cannot.
This happens because water molecules are small enough to fit through the holes in the eggshell, while larger particles of calcium carbonate do not fit.

What Happens During Diffusion?
Diffusion describes how molecules spread apart and mix into each other when they’re in different concentrations.
These molecules will spread apart and mix with each other in order to equalize the concentration.
This occurs because of random movement – if two molecules are close together but have different concentration levels, the concentration level in the vicinity may change due to diffusion over time.
What is Chemical Reaction?
A chemical reaction usually changes the state of substances.
As a result of this process new substances, called products, are produced.
Usually, one or more types of molecules are involved in chemical reactions.
In this science experiment, when vinegar reacts with calcium carbonate, two new substances are produced – carbon dioxide gas and calcium acetate.
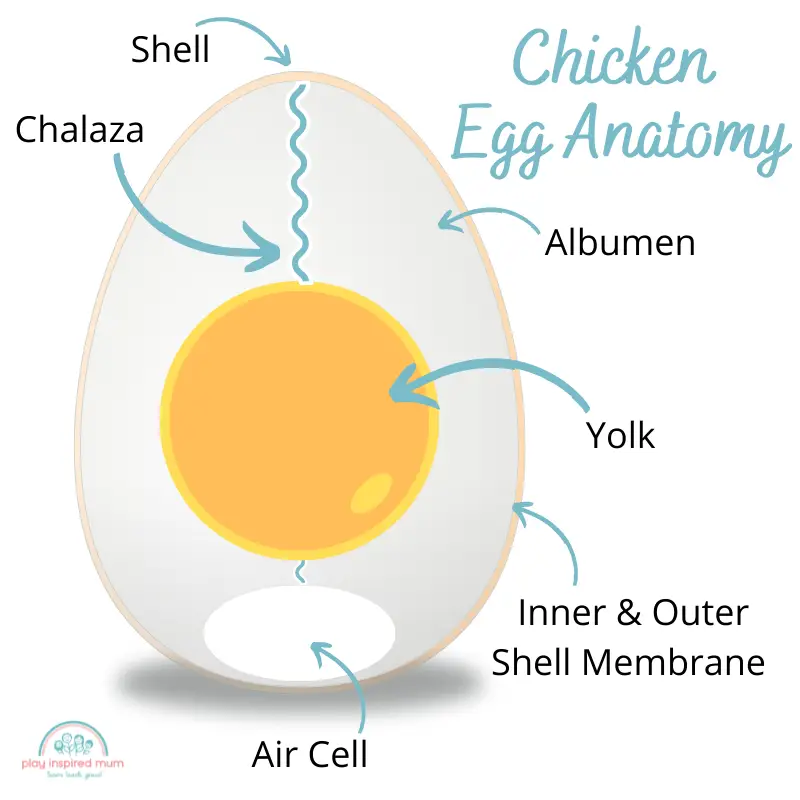
Layers of an egg
An egg is made up of several layers, including the shell, the albumen (or egg white), and the chalaza.
The eggshell is made up of calcium carbonate, which is why it dissolves in vinegar.
The albumen contains protein and water, while the chalaza is a cord that attaches the yolk to the albumen.
The eggshell protects the egg from bacteria and other contaminants, while the albumen provides food and energy for the embryo. The chalaza helps to move the yolk around inside the egg.
What is an Eggshell Made of?
The shell of an egg is composed of tough, flexible plates made mostly from calcium carbonate. There are also cells and membranes throughout the shell. This helps protect and support the developing baby chick inside.
What Happens When Vinegar Meets the Egg Shell?
When the egg is soaked in vinegar, the acetic acid (in the vinegar) reacts with the calcium carbonate shell of the egg.
Diffusion and osmosis work on eggshells like all other cells in living organisms.
Cells lining an eggshell are surrounded by a membrane that separates them from their surrounding environment.
The membrane is semi-permeable, which means that it mostly only lets water through the membrane.
This is why when you crack a boiled egg open you find a membrane between the egg white and the shell.
The center part of the egg contains more water than the rest of the egg.
When an egg is soaked in vinegar, diffusion and osmosis work together to change the egg’s chemical composition over time by moving water outward into the acetic acid solution.
As more and more water moves out of the cell walls due to osmosis, eventually they burst because there’s not enough water left inside them.
At this point, all that’s left are dried-up pieces of the membrane lining the inside of your shell.
These can be gently rinsed away.
In this reaction, hydrogen ions from the acetic acid react with the calcium carbonate shell of the egg to produce water and solid products of carbon dioxide.
Once the shell is gone, the vinegar pickles the outer membrane as it crosses over via osmosis.
This toughens the egg, giving it a bit of a bounce come to the end of the experiment. It also is what causes the egg to swell.

What is the chemical reaction?
The reaction equation that has occurred is
CaCO3 + 2CH3COOH → Ca(CH3COO)2 + CO2↑ + H2O
How long does the rubber egg experiment take?
The reaction involved in the rubber egg science experiment is noticeable instantly.
Bubbles or the carbon dioxide will start forming straight away.
The shell of the egg is typically visibly compromised with a day.
The shell is usually dissolved by day three.
After a week the membrane has toughened the egg enough to render the egg rubbery or bouncy.
Although we call the egg a rubber egg, the centre is still liquid. Remember to handle the rubber egg with care.

Will the rubber egg bounce?
The rubber egg will bounce from low heights. Start just a couple of centremetres from the bench and increase to discover your eggs threshold! This is an experiment to do over the sink or outside… It will end with the egg bursting!
Our egg was able to be dropped from a height of about 30cm however we have read of eggs being dropped without breaking from a height of 50cm!
Variations of the experiment
There are many different variations of the rubber egg experiment that you can try.
Once your child understands the fundamentals of the experiment, why not experiment with a few variables?
For example, you could try using different types of vinegar, such as
- white vinegar
- apple cider vinegar
- balsamic vinegar
Would other acidic liquids work the same?
- Lemon juice
- Soft drink
- Tomato
Egg variations
You could also try using different types of eggs such as
- chicken eggs
- duck eggs
- goose eggs
- quail eggs
Add art
Create a design using a wax crayon or candle to see if the wax will protect the shell.
Hypothetically the shell would be protected by the wax so not in contact with the vinegar. Will the wax hold up over the duration of the experiment?
Temperature
Try experimenting with different temperatures to observe how it impacts the result.
Store one experiment in the fridge and another at room temperature and compare the results.
There are so many variations to this experiment.
Which are you going to try first?